The Science Behind Spermidine
Spermidine
Spermidine, what an intriguing name! Don’t you panic though! Spermidine may have first been detected in sperm but this is not where it is sourced from. In fact, spermidine is a ubiquitous molecule in all organisms (1). The richest dietary sources of spermidine are plants, especially soybean oil and wheat germ, and mushrooms (2). Good animal sources are seafood and liver (3). Even our gut flora produces small amounts of spermidine (4).
Spermidine belongs to the family of natural polyamines. Polyamines are molecules with multiple amino groups (NH) (Figure 1). They are famous for their roles in cell proliferation, gene regulation, and more (5). Spermidine has recently been the center of a lot of attention, with 23 trials registered at clinicaltrials.gov. Out of these, two trials already have results and along with epidemiological and animal studies have formed our knowledge on the benefits of spermidine.
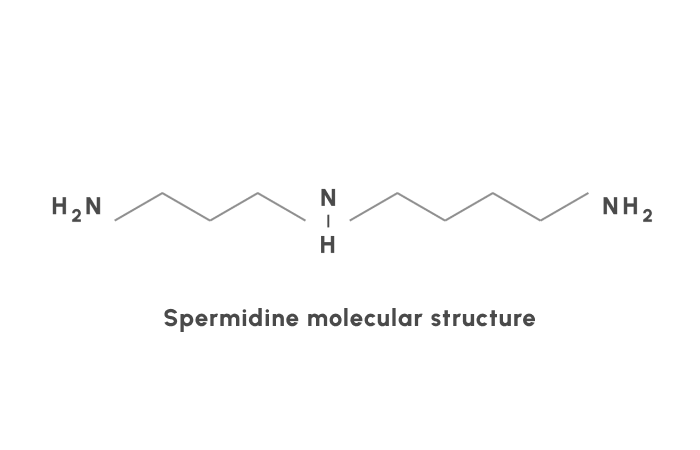
Figure 1: The structure of spermidine. Spermidine is a natural polyamine with three amino (NH) groups.
Spermidine boosts autophagy
Inducing autophagy is one of the most essential roles of spermidine (6). The word autophagy means self-devouring in greek. That may not sound pleasant but autophagy is vital for survival and growth. During autophagy, the cell removes and recycles material that is unnecessary or harmful. So, autophagy contributes to the nutrition and “maintenance” of each cell and the body as a whole. Defects in autophagy are linked to diseases and aging (7). To illustrate, if for some reason autophagy in our cells stopped, we would die shortly after (8). Spermidine induces autophagy by regulating the expression of autophagy-related genes (9). This is a different mechanism than that of resveratrol and the two molecules may have synergistic effects (10). This ability of spermidine to promote autophagy is the cornerstone of most of its health benefits.
It reduces inflammation
Chronic inflammation is a driver of aging and there is even a term for it: “inflammaging” (11). With age, pro-inflammatory markers rise and so does the risk of age-related conditions. These include frailty, cognitive dysfunction, cardiovascular diseases, diabetes, and multi-morbidity (11). The decline of autophagy with age further contributes to chronic inflammation (12).
Spermidine has potent anti-inflammatory properties. In vivo studies demonstrate that it suppresses pro-inflammatory cytokines like TNF-α, IL-1β and IL-18 (13–15). It also protects from the pro-inflammatory transcription factor NF-κΒ and the buildup of reactive oxygen species in zebrafish (14). With these mechanisms, along with boosting autophagy, spermidine could downregulate systemic, chronic inflammation.
It may promote longevity
Weakened autophagy and chronic inflammation are both drivers of aging. By fighting those factors spermidine holds great promise for longevity. Indeed, spermidine increased the lifespan of several species and human immune cells (13,16,17). These animals also showed better autophagy and mitochondrial function (13). In a similar manner, flies and mice with overactivation of autophagy genes live longer (18,19).
Good news comes from some human studies, too. According to an observational study, people who lived longer had polyamine (including spermidine) levels similar to middle-aged people (20). What’s more, a hallmark prospective study with 829 participants concluded that people who received more dietary spermidine had lower mortality (21). Although the general effect of a good diet cannot be excluded, these studies show that higher spermidine correlates with longer life. More research is needed to prove if this is a cause-and-effect relationship.
It protects the cardiovascular system
The cardiovascular system is detrimentally affected by chronic inflammation (11). Therefore, it comes as no surprise that spermidine can protect cardiovascular function (6). Several in vivo studies show that spermidine lessens high blood pressure and atherogenesis (the building up of material in the arteries) and overall protects heart function (22,23). Furthermore, spermidine was shown to mitigate cardiac aging by enhancing mitochondrial function. The mechanism behind that was greater sirtuin activity and NAD levels (24).
Human studies are not missing in this field, either. Epidemiological studies report that a higher intake of natural polyamines, including spermidine, correlates with a smaller risk for cardiovascular diseases like coronary artery disease, hypertension, and atherosclerosis (21,25). In a randomized control trial, participants received yoghurt with Bifidobacterium probiotics and arginine. The probiotics in the gut produced spermidine and putrescine (another polyamine). Three months later, the participants had better endothelial function and a smaller risk for atherosclerosis (26). Overall, there is a lot of research supporting the great cardiovascular benefits of spermidine.
Spermidine supports brain health
Polyamines, including spermidine, play an important role in the nervous system. Imbalances in their metabolism are linked to strokes as well as neurodegenerative diseases like Alzheimer’s and Parkinson’s (27,28). For example, in a recent human study, higher blood levels of spermidine were correlated to a higher risk of stroke (29). The mechanism behind this is unclear, and a causative relationship is not proven.
It may seem contradictory but supplementation of spermidine seems to have positive results in brain health. In mice it improved mitochondrial function and autophagy and, as a result, it delayed brain aging (30). Similar results produced a pilot human randomized control trial. Older people at risk of Alzheimer’s received 750 mg of spermidine-rich plant extract for three months. By the end of the study, participants saw improvements in memory and cognitive function (31). In yet another human study, spermidine was related to better structural brain measures and enhanced the positive effect of a Mediterranean diet (32).
Spermidine supports metabolic health
Spermidine may have positive effects on metabolic syndrome and obesity (33). These effects are likely mediated by autophagy, which contributes to weight loss when in starvation. By enhancing autophagy, spermidine can help with weight loss. Spermidine not only mitigated the gain weight in animals on a hypercaloric diet but also protected them from increased glucose tolerance and insulin sensitivity. These health benefits were attributed to enhanced autophagy in fat tissue (34). In a similar study, spermidine alleviated some more health problems of mice in a high-fat diet: it reduced inflammation, strengthened the gut barrier, reduced fat mass and improved insulin resistance (35,36). These results are promising and human research could determine if spermidine would consist a suitable treatment for metabolic health problems.
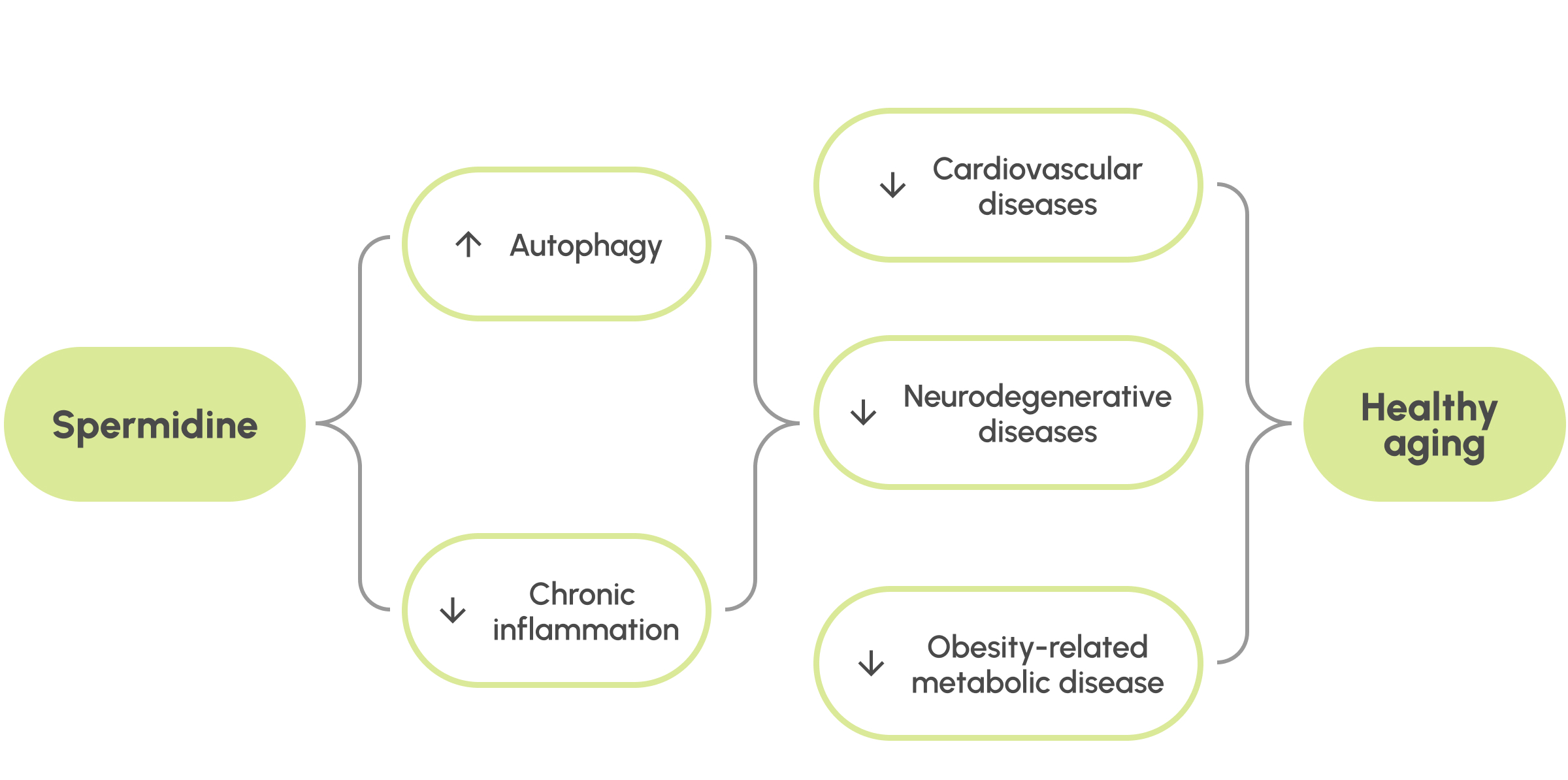
Figure 2: Potential health benefits of spermidine.
What we still need to find out
Due to its central role in metabolism, spermidine is interconnected in many pathways and some of its aspects remain unclear. For example, high spermidine levels are observed in diseases like neurodegenerative diseases and cancer, without that meaning that it causes the disease (33). In fact, the data on spermidine supplementation and cancerogenesis remain contradicting. We can expect that future research will shed light into these secrets.
Conclusion
Spermidine is an important molecule in metabolism and essential for promoting autophagy and controlling inflammation. Some preliminary results in animals and humans also support benefits for cardiovascular, neurodegenerative and metabolic health diseases. Some studies even show a potential connection with longevity. There is still a lot to find out about the action and benefits of spermidine and the human trials that are underway promise to do just that.
References
1. Ali MA, Poortvliet E, Strömberg R, Yngve A. Polyamines in foods: development of a food database. Food Nutr Res 2011;55.
2. Madeo F, Hofer SJ, Pendl T, Bauer MA, Eisenberg T, Carmona-Gutierrez D, et al. Nutritional Aspects of Spermidine. Annu Rev Nutr 2020;40:135–59.
3. Zou D, Zhao Z, Li L, Min Y, Zhang D, Ji A, et al. A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Compr Rev Food Sci Food Saf 2022;21:2820–42.
4. Ramos-Molina B, Queipo-Ortuño MI, Lambertos A, Tinahones FJ, Peñafiel R. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Front Nutr 2019;6:24.
5. Bae DH, Lane DJR, Jansson PJ, Richardson DR. The old and new biochemistry of polyamines. Biochim Biophys Acta Gen Subj 2018;1862:2053–68.
6. Ni YQ, Liu YS. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis 2021;12:1948.
7. Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nature Reviews Molecular Cell Biology 2018 19:9 2018;19:579–93.
8. Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha S v., Khor S, et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 2014;4:915–27.
9. Morselli E, Mariño G, Bennetzen M v., Eisenberg T, Megalou E, Schroeder S, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 2011;192:615.
10. Morselli E, Mariño G, Bennetzen M v., Eisenberg T, Megalou E, Schroeder S, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 2011;192:615.
11. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505.
12. Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166.
13. Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nature Medicine 2016 22:12 2016;22:1428–38.
14. Jeong JW, Cha HJ, Han MH, Hwang SJ, Lee DS, Yoo JS, et al. Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol Ther (Seoul) 2018;26:146–56.
15. Xu TT, Li H, Dai Z, Lau GK, Li BY, Zhu WL, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020;12:6401–14.
16. Eisenberg T, Abdellatif M, Zimmermann A, Schroeder S, Pendl T, Harger A, et al. Dietary spermidine for lowering high blood pressure.
17. Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nature Cell Biology 2009 11:11 2009;11:1305–14.
18. Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 2013;4.
19. Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep 2014;8:1767–80.
20. Pucciarelli S, Moreschini B, Micozzi D, de Fronzo GS, Carpi FM, Polzonetti V, et al. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res 2012;15:590–5.
21. Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr 2018;108:371–80.
22. Tyrrell DJ, Blin MG, Song J, Wood SC, Zhang M, Beard DA, et al. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ Res 2020;126:298.
23. Eisenberg T, Abdellatif M, Zimmermann A, Schroeder S, Pendl T, Harger A, et al. Dietary spermidine for lowering high blood pressure. Autophagy 2017;13:767.
24. Wang J, Li S, Wang J, Wu F, Chen Y, Zhang H, et al. Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging (Albany NY) 2020;12:650.
25. Soda K, Kano Y, Chiba F. Food Polyamine and Cardiovascular Disease -An Epidemiological Study-. Glob J Health Sci 2012;4:170.
26. Matsumoto M, Kitada Y, Naito Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, Vol 11, Page 1188 2019;11:1188.
27. Cervelli M, Averna M, Vergani L, Pedrazzi M, Amato S, Fiorucci C, et al. The Involvement of Polyamines Catabolism in the Crosstalk between Neurons and Astrocytes in Neurodegeneration. Biomedicines 2022;10:1756.
28. Park MH, Igarashi K. The polyamines, putrescine, spermidine and spermine: 3 Polyamines and Their Metabolites as Diagnostic Markers of Human Diseases 2013.
29. Zheng L, Xie Y, Sun Z, Zhang R, Ma Y, Xu J, et al. Serum Spermidine in Relation to Risk of Stroke: A Multilevel Study. Front Nutr 2022;9:496.
30. Xu TT, Li H, Dai Z, Lau GK, Li BY, Zhu WL, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020;12:6401–14.
31. Wirth M, Benson G, Schwarz C, Köbe T, Grittner U, Schmitz D, et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 2018;109:181–8.
32. Schwarz C, Horn N, Benson G, Wrachtrup Calzado I, Wurdack K, Pechlaner R, et al. Spermidine intake is associated with cortical thickness and hippocampal volume in older adults. Neuroimage 2020;221.
33. Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science (1979) 2018;359.
34. Fernández ÁF, Bárcena C, Martínez-García GG, Tamargo-Gómez I, Suárez MF, Pietrocola F, et al. Autophagy couteracts weight gain, lipotoxicity and pancreatic β-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis 2017;8.
35. Ma L, Ni Y, Hu L, Zhao Y, Zheng L, Yang S, et al. Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in preexisting obese mice. Life Sci 2021;265.
36. Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F, et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020;12:1–19.
Spermidine Quality Reports
All of our product has been manufactured in a US-based, FDA, GMP, and NSF registered facility under strict GMP standards, and third-party lab tested.